EPic Pulmonary Smartphrases
Alison Lee
Esbriet Dosing for Idiopathic Pulmonary Fibrosis
Initial dose titration
- Take with food
- Days 1-7: 267 mg (1 capsule) by mouth three times daily
- Days 8-14: 534 mg (2 capsules) by mouth three times daily
- Day 15 and thereafter: 801 mg (3 capsules) by mouth three times daily
- Maintenance dose
- 801 mg (3 capsules) by mouth three times daily
- Not to exceed 2403 mg/day (9 capsules/day)
- Labs
- Every 2 weeks for first month while titrating medication dose
- Every month for first 3 months
- Every 3 months to follow
Pirfenidone (Esbriet):
Pirfenidone (PF) is an orally active, anti-fibrotic and anti-inflammatory agent that inhibits the synthesis of transforming growth factor (TGF-beta), a chemical mediator that controls many cell functions including proliferation and differentiation, and plays a key role in fibrosis. It also inhibits the synthesis of tumor necrosis factor (TNF-alpha), a cytokine that is known to have an active role in inflammation. In addition to baseline routine labs (CBC, BMP, LFT) we recommend a baseline pre-treatment test for TB (with Quantiferon Gold), Hep B and Hep C.
PF is usually given orally with food as follows:
Initial dose titration:
Days 1-7: 267 mg (1 capsule) by mouth three times daily
Days 8-14: 534 mg (2 capsules) by mouth three times daily
Day 15 and thereafter: 801 mg (3 capsules) by mouth three times daily
Maintenance dose:
Usually 801 mg (3 capsules) by mouth three times daily (not to exceed 2403 mg/d or 9 capsules/d).
It is recommended to conduct liver function tests (ALT, AST, and bilirubin) before initiating, monthly for 6 months, and then every 3 months thereafter. We also recommend other routine blood tests (CBC, BMP) every 2 wks while escalating therapy, every month x 3 and then every 3 months if stable. A pre-treatment ECG is also recommended.
Dose adjustment is recommended as follows:
If AST/ALT >3 to ≤5 x ULN (without symptoms): Discontinue confounding medications, exclude other causes, repeat liver function tests as needed; the full daily dosage may be maintained, if clinically appropriate, or reduced or interrupted (until liver chemistry tests are within normal limits), with subsequent re-titration to the full dosage as tolerated.
If AST/ALT >5 x ULN or >3 times ULN with signs/symptoms of severe liver damage: Permanently discontinue; do not re-challenge.
For renal impairment: mild, moderate, or severe: consider dosage modification and for ESRD requiring dialysis: No recommended (not studied).
Common GI side effects are nausea, vomiting, diarrhea, indigestion, heartburn, and pain. If patients experience significant adverse reactions (ie, gastrointestinal, photosensitivity reaction, rash), we consider temporary dosage reductions or therapy interruptions to allow for resolution of symptoms. Since serious side effects include liver toxicity, patients are instructed to call their MD for jaundice, dark-colored urine, pain on the right upper quadrant of abdomen, bleeding or bruising, or feeling tired. The use of PF is contraindicated with carbamazepine, phenobarbital, primidone and rifampin.
There are serious drug-drug interactions with fluoroquinolones, amiodarone, amlodipine/nifedipine, cimetidine, diclofenac, gemfibrozil and zileuton. Post marketing reports of agranulocytosis and angioedema have surfaced. PF is considered a category C drug in pregnancy (Pregnancy Category C: Use with caution if benefits outweigh risks. Animal studies show risk and human studies not available or neither animal nor human studies done).
Due to the adverse effects of photosensitivity and rash patients are instructed to avoid unnecessary or prolonged exposure to real and artificial sunlight (tanning beds, sunlamps) and light therapy and wear protective clothing, sunglasses, and sunscreen (with a SPF factor of 30 or above).
General Information
Ofev (nintedanib) is a small molecule kinase inhibitor that blocks multiple pathways that may be involved in the scarring of lung tissue.
Ofev is specifically indicated for the treatment of idiopathic pulmonary fibrosis.
Ofev is supplied as a capsule for oral administration. The recommended dosage of Ofev is 150 mg twice daily administered approximately 12 hours apart. Ofev capsules should be taken with food and swallowed whole with liquid.
Clinical Results
FDA Approval
The FDA approval of Ofev was based on the results of one phase II and two phase III studies. These randomized, double-blind, placebo-controlled studies enrolled a total of 1,231 subjects and compared treatment with Ofev 150 mg twice daily to placebo for 52 weeks. The subjects were randomized to either Ofev 150 mg or placebo twice daily for 52 weeks. The phase II study also included other treatment arms (50 mg daily, 50 mg twice daily, and 100 mg twice daily). The primary endpoint was the annual rate of decline in Forced Vital Capacity (FVC). A statistically significant reduction in the annual rate of decline of FVC (in mL) was demonstrated in patients receiving OFEV compared to patients receiving placebo based on the random coefficient regression model, adjusted for gender, height, and age.
Side Effects
Adverse effects associated with the use of Ofev may include, but are not limited to, the following:
- diarrhea
- nausea
- abdominal pain
- vomiting
- liver enzyme elevation
- decreased appetite
- headache
- weight decreased
- hypertension
- Mechanism of Action
- Ofev (nintedanib) is a small molecule that inhibits multiple receptor tyrosine kinases (RTKs) and non-receptor tyrosine kinases (nRTKs). Nintedanib inhibits the following RTKs: platelet-derived growth factor receptor (PDGFR) α and β, fibroblast growth factor receptor (FGFR) 1-3, vascular endothelial growth factor receptor (VEGFR) 1-3, and Fms-like tyrosine kinase-3 (FLT3). Among them, FGFR, PDGFR, and VEGFR have been implicated in IPF pathogenesis. Nintedanib binds competitively to the adenosine triphosphate (ATP) binding pocket of these receptors and blocks the intracellular signaling which is crucial for the proliferation, migration, and transformation of fibroblasts representing essential mechanisms of the IPF pathology. In addition, nintedanib inhibits the following nRTKs: Lck, Lyn and Src kinases. The contribution of FLT3 and nRTK inhibition to IPF efficacy is unknown.
M. abscessus lung disease may progress very slowly; furthermore, some patients do not require treatment, whereas others require combination antibiotic therapy, including parenteral agents. After discussing this information with the patients, we implemented an observation period of at least 6 to 12 months without antibiotic treatment. When the disease was clearly recognized as being progressive, the patients received a standardized combination antibiotic therapy after hospitalization. In patients with substantial symptoms and/or advanced or progressive radiographic abnormalities, antibiotic therapy was initiated immediately.
A clarithromycin-containing three-drug oral regimen that included clarithromycin (1,000 mg/d), ciprofloxacin (1,000 mg/d), and doxycycline (200 mg/d), along with an initial 4-week course of amikacin (15 mg/kg/d in two divided doses) and cefoxitin (200 mg/kg/d, maximum 12 g/d in three divided doses) Peak serum levels of amikacin (> 20 μg/ml) were achieved using therapeutic drug monitoring. Complete blood cell counts, serum creatinine, and liver function test results were monitored twice a week during hospitalization. If an adverse reaction associated with cefoxitin occurred, imipenem (750 mg, three times a day) (3) was substituted for cefoxitin.
After discharge, patients took a three-drug oral regimen for a total treatment duration of 24 months. This regimen continued for at least 12 months after sputum culture conversion. Sputum smear and culture examinations were performed monthly for the first 6 months and then at 2- to 3-month intervals until the end of treatment.
Measurements and Main Results: Treatment response rates were 83% for symptoms and 74% for high-resolution computed tomography. Sputum conversion and maintenance of negative sputum cultures for more than 12 months was achieved in 38 (58%) patients. These rates were significantly lower in patients whose isolates were resistant to clarithromycin (17%, 2/12) compared with those whose isolates were susceptible or intermediate to clarithromycin (64%, 21/33; P = 0.007). Neutropenia and thrombocytopenia associated with cefoxitin developed in 33 (51%) and 4 (6%) patients, respectively. Drug-induced hepatotoxicity occurred in 10 (15%) patients. Because of these adverse reactions, cefoxitin was discontinued in 39 (60%) patients after treatment for a median of 22 days.
Conclusions: Standardized combination antibiotic therapy was moderately effective in treating M. abscessus lung disease. However, frequent adverse reactions and the potential for long-duration hospitalization are important problems that remain to be solved.
Treatment.
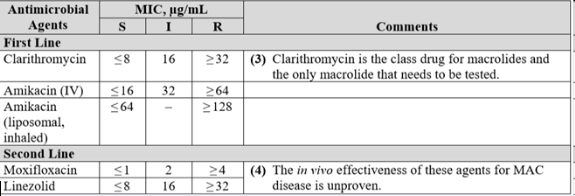
M. abscessus isolates are uniformly resistant to the standard antituberculous agents (358–360). Because of variable in vitro drug susceptibilities to some drugs, antibiotic susceptibility testing of all clinically significant isolates is recommended. Acquired mutational resistance to clarithromycin (23S rRNA gene) and amikacin (16S rRNA gene) can occur because isolates of M. abscessus have only a single copy of the gene. Untreated M. abscessus isolates generally have low or intermediate MICs, compared with achievable drug levels, to clarithromycin (100%), amikacin (90%), and cefoxitin (70%). Some isolates have low or intermediate MICs to linezolid. Some isolates (50%) also show low MICs to imipenem, although, as noted, this determination is not reliable (see Laboratory Procedures). Isolates also have low MICs to clofazimine, although methodology and MIC breakpoints have not yet been addressed by the CLSI for this agent.
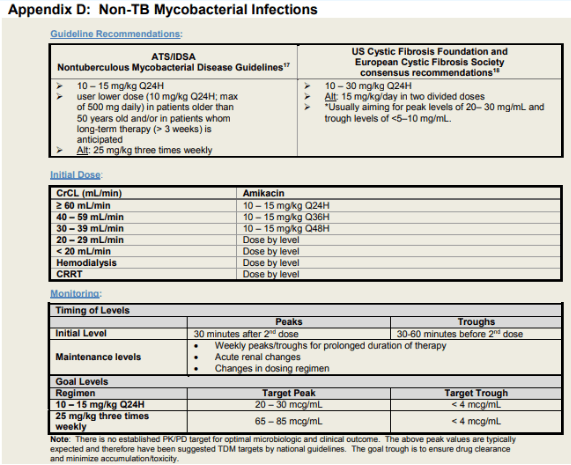
For serious skin, soft tissue, and bone infections caused by M. abscessus, clarithromycin 1,000 mg/day or azithromycin 250 mg/day should be combined with parenteral medications (amikacin, cefoxitin, or imipenem). The macrolides are the only oral agents reliably active in vitro against M. abscessus (359, 361). The most active of the parenteral agents is amikacin. Intravenous amikacin is given at a dose of 10 to 15 mg/kg daily to adult patients with normal renal function to provide peak serum levels in the low 20-mg/ml range. Once-daily dosing is unproven clinically but appears reasonable. The lower dose (10 mg/kg) should be used in patients older than 50 years and/or in patients in whom long-term therapy (> 3 wk) is anticipated. The three-times-weekly amikacin dosing at 25 mg/kg is also reasonable, but may be difficult to tolerate over periods longer than 3 months (297). The amikacin combined with high-dose cefoxitin (up to 12 g/d given intravenously in divided doses) is recommended for initial therapy (minimum, 2 wk) until clinical improvement is evident. Limited cefoxitin availability may necessitate the choice of an alternative agent such as imipenem (500 mg two to four times daily), which is a reasonable alternative to cefoxitin (175, 359, 360). For serious disease, a minimum of 4 months of therapy is necessary to provide a high likelihood of cure. For bone infections, 6 months of therapy is recommended (354). Surgery is generally indicated with extensive disease, abscess formation, or where drug therapy is difficult. Removal of foreign bodies, such as breast implants or percutaneous catheters, is important and probably essential to recovery.
In contrast to the efficacy of medication regimens for nonpulmonary disease, no antibiotic regimens based on in vitro susceptibilities has been shown to produce long-term sputum conversion for patients with M. abscessus lung disease. The goal of 12 months of negative sputum cultures while on therapy may be reasonable, but there is no medication strategy to reliably achieve this goal. Alternative goals of therapy, such as symptomatic improvement, radiographic regression of infiltrates, or improvement in sputum culture positivity, short of conversion to negative culture, are more realistic at this point for M. abscessus lung disease. Monotherapy with macrolides is not sufficient to produce microbiologic cure for M. abscessus lung disease. Combination therapy (as outlined above) with amikacin plus cefoxitin or imipenem for 2 to 4 months usually produces clinical and microbiologic improvement, but cost and morbidity are significant impediments to a curative course of therapy. For patients with macrolide-resistant M. abscessus isolates or macrolide intolerance, most experts would recommend a combination of parenteral drugs based on in vitro susceptibilities. For some patients, symptoms can be controlled with intermittent periods of therapy with clarithromycin or azithromycin alone or in combination with one or more parenteral drugs. Suppressive therapy, including periodic parenteral antibiotic or oral macrolide therapy, may be all that can be realistically administered to control the symptoms and progression of M. abscessus lung disease. Because side effects and toxicities are common with aggressive parenteral therapy, expert consultation is recommended for these patients. Overall, with current antibiotic options, M. abscessus is a chronic incurable infection for most patients.
Curative therapy for M. abscessus lung disease is more likely to be obtained with limited disease and a combination of surgical resection of involved lung and chemotherapy (32). Patients with focal lung disease who can tolerate lung resection should be treated with surgery after an initial period on antimicrobial drug therapy to lessen the microbial burden. Caveats discussed for MAC lung disease surgery are pertinent for M. abscessus as well. For patients with underlying esophageal or other swallowing disorders, treatment of the underlying condition can result in improvement of the RGM lung disease.
Drugs that show some potential but are not extensively tested include three newer classes of drugs, the oxazolidinones, the glycylcyclines, and the ketolides, that all have some in vitro activity against M. abscessus (362). Approximately 50% of M. abscessus isolates are susceptible or intermediate in susceptibility in vitro to linezolid, the first FDA-approved oxazolidinone (363). A small number of patients with M. abscessus lung disease have been treated with linezolid and a companion drug, usually a macrolide, with mixed results. Long-term linezolid therapy at usually recommended antibacterial doses (600 mg twice daily) is often associated with severe side effects, such as anemia, peripheral neuropathy, nausea, and vomiting. A smaller dose, 600 mg/day, is associated with fewer gastrointestinal and hematologic side effects and may still have significant antimycobacterial activity (363). The tetracycline derivatives, glycylcyclines, especially tigecycline, also have in vitro activity against M. abscessus. This drug must be given intravenously and it is known to cause nausea and anorexia in some patients when given long term for mycobacterial disease. Telithromycin, a ketolide, in limited testing has in vitro activity against some M. abscessus isolates but has not been evaluated clinically.
Context:1.At present, there is no reliable or dependable antibiotic regimen, even based on in vitro susceptibilities and including parenteral agents, to produce cure for M. abscessus lung disease.Recommendations:1.The only predictably curative therapy of limited (focal) M. abscessus lung disease is surgical resection of involved lung combined with multidrug chemotherapy (A, II).2.Periodic administration of multidrug therapy, including a macrolide and one or more parenteral agents (amikacin, cefoxitin, or imipenem) or a combination of parenteral agents over several months may help control symptoms and progression of M. abscessus lung disease (C, III).
The total ACT score is based on a range of 5 to 25. A score of 19 or less may be a sign that asthma symptoms are not under control. If patients score 19 or less, they should meet with their physician to discuss their ACT results and ensure they are properly controlling airway constriction and inflammation. Experts also recommend that even if patients score >20 they should talk with their physician about their results to help improve their asthma.
American Lung Association is recommending the new, scored Asthma Control Test (ACT) for all asthma patients 12 years and older. The test is a 5-question assessment tool, which provides physicians and patients a simple yet highly predictive tool they can use to help assess asthma control. The questions included in the test are based on measures of asthma control established by the National Institutes of Health.
The total ACT score is based on a range of 5 to 25. A score of 19 or less may be a sign that asthma symptoms are not under control. If patients score 19 or less, they should meet with their physician to discuss their ACT results and ensure they are properly controlling airway constriction and inflammation. Experts also recommend that even if patients score >20 they should talk with their physician about their results to help improve their asthma.
Adempas (Riociguat)
Recommended starting dose:
Start with 1 mg taken 3x a day
For patients who may not tolerate the hypotensive effects of Adempas, consider a starting dose of 0.5 mg taken 3x a day
Titration through maintenance
Individualized dose adjustments
If systolic blood pressure remains >95 mm Hg and the patient has no signs or symptoms of hypotension, up-titrate the dose by 0.5 mg. Dose increases should be no sooner than 2 weeks apart. The dose can be increased to the highest tolerated dosage, up to a maximum of 2.5 mg taken 3x a day
If at any time the patient has symptoms of hypotension, decrease the dosage by 0.5 mg
Your physician has referred you for allergy skin prick testing for environmental (e.g. pollen, dust mites, mold, pet dander) or for food allergens.
You need to stop the following medications prior to the first visit so that this allergy skin prick test can be performed on that visit (if indicated).
Oral antihistamines (e.g. Benadryl, Zyrtec, Claritin, Allegra, Zyzal) 5 day prior to allergy visit.
Intranasal (e.g. Astelin, Patanase) and eye drop antihistamines (e.g. Zaditor, Patanol) 2 days prior to allergy visit.
Benzodiazepines (if possible) (e.g. Klonopin, Xanax) 1 week prior to allergy visit.
It’s ok to continue Singulair and Flonase and other inhalers you may be taking.
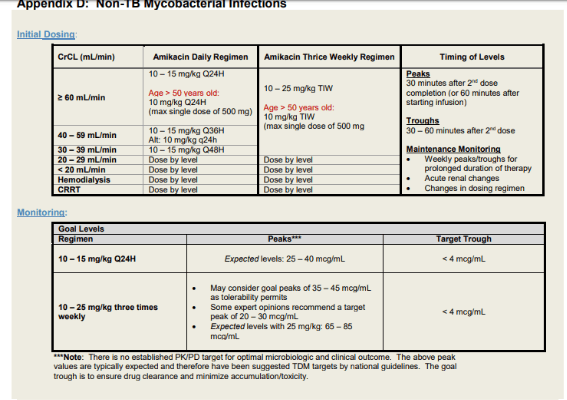
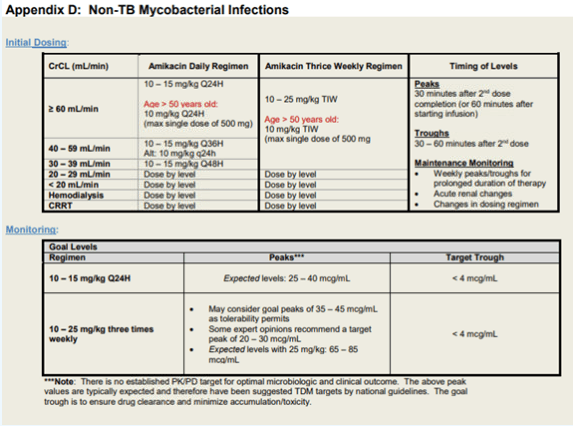
Amikacin
Intravenous amikacin for MAC
General dosing recommendation
· amikacin IV 15-20 mg per kg three times a week
Target peak: 35-45
· commence after completion of second dose of amikacin
· draw peak level 30 min after end of the infusion
· check for the first 2 weeks of treatment
Target trough: undetectable (<2 or <4)
· commence after completion of second dose of amikacin
· draw trough level 30 mins prior to infusion
· monitor weekly while on treatment with amikacin
For patient with higher amikacin MIC, discussion case by case as a team for target peaks
Clinical screening for ocular and oto-vestibular toxicity at each clinic visit (Ishihara charts, Romberg test)
Baseline audiology visit and then monthly while on IV amikacin with last examination 2 months after end of IV amikacin course
Baseline ocular visit if starting ethambutol and then as per recommendations of ophthalmologist depending on initial findings
Baseline EKG for QTc, follow up in 2 weeks of starting antibiotics and then every 3-6 months if stable.
Continue monthly audiology testing monthly while on inhaled amikacin
Recommend amikacin trough level at least once on everyone
We discussed using Budesonide-formoterol (Symbicort) or mometasone-formoterol (Dulera) as needed for mild asthma or SMART (SINGLE MAINTENANCE AND RELIEVER) for moderate asthma
- If using as needed for mild asthma you can use 1-2 puffs as needed for asthma symptoms up to a maximum of 12 puffs (54 mcg formoterol) daily
- If you are taking a daily maintenance dose you can take one to two puffs once or twice daily and then take additional rescue doses of one to two puffs as needed for asthma symptoms, up to a maximum of 12 puffs daily of either daily maintenance dose and relief doses (54 mcg formoterol)
- If you are taking 12 puffs daily for 2 days in a row, please reach out to your asthma provider for further instructions
- Please know that using budesonide formoterol or mometasone formoterol this was is not US FDA approved but is preferred recommended therapy for mild and moderate asthma based on USA NAEPPR 4 guidelines and Global Initiative for Asthma has been found to be safe and effective in many studies
- A reminder as discussed with your prescriber that the instructions on your prescriptions and the information that comes with your inhaler may differ from the instructions you have been given
- If you have any questions or are not sure what to do, please reach out to your provider for further instructions
Inhaler Use
The inhaler that you were prescribed contains a potent medication. It should only be used as directed. The medicine in your inhaler must be breathed deeply into your lungs in order for it to work. It will not work at all if it only reaches your mouth and throat. Follow the instructions below for best results. And remember to follow your Asthma Action Plan as given to you by your doctor.
- Keep your inhaler at room temperature.
- Hold the inhaler so that the part that goes into your mouth is at the bottom.
- Shake the inhaler well and remove the cap.
- Breathe out fully through your mouth.
- Place the inhaler in your mouth and close your lips tightly around it. (Or hold the inhaler 1 to 2 inches from your open mouth if told to do so by your healthcare provider.)
- Squeeze the inhaler as you breathe in slowly through your mouth for a few seconds, drawing the medicine deep into your lungs.
- Hold your breath for up to10 seconds. Then breathe out slowly.
- If you have been advised to take two puffs, wait one minute, then repeat steps 3-7 above. Replace the cap when done.
- If you were prescribed both a steroid inhaler and a bronchodilator inhaler, use the bronchodilator first to open the air passages. Wait 5 minutes, then use the steroid inhaler.
- Rinse your mouth with water and spit it out (especially after using a steroid inhaler)
- A special chamber (“spacer”) may be prescribed that attaches to your inhaler (Aerochamber and other brands). This increases the amount of medicine that goes to your lungs. It also improves how well each treatment works. Ask your doctor about this if you did not receive one.
- Keep It Clean
- Remove the metal canister and do not immerse it in water. Then clean the plastic mouthpiece, cap, and spacer if you have one, by rinsing them well in warm running water for 30 to 60 seconds. Shake off excess water and allow the mouthpiece to dry completely (overnight is recommended). If you need the inhaler before the mouthpiece is dry, shake off excess water, replace canister, and test spray two times (away from the face). This should be done once each day of use. This is very important if you are using a steroid inhaler in order to prevent thrush. This is an infection that occurs in the mouth.
- Warning
- A steroid inhaler is used to prevent an asthma attack. Do not use this to treat an acute wheezing episode. It might even make an acute attack worse. Use only bronchodilator inhalers to treat an acute asthma attack (albuterol, Vanceril, Proventil, and others).
- If you find that your medicine is not working and you need to use it more often than prescribed, this could be a sign that your asthma is getting worse. Go to the emergency room or urgent care right away. An asthma attack is easiest to treat in the early stages before it becomes severe!
- Get Prompt Medical Attention
- Get prompt medical attention if any of the following occur:
- Increased wheezing or shortness of breath
- Need to use your inhalers more often than usual without relief
- Fever of 100.4°F (38°C) or higher, or as directed by your health care provider
- Coughing up lots of dark-colored or bloody sputum (mucus)
- Chest pain with each breath
- Blue lips or fingernails
- Peak flow reading less than 50 percent of your normal best

Stepwise Approach:
For persistent asthma (step 2 through step 6)
Consult with asthma specialist if step 4 care or higher is required. Consider consultation
at step 3.
| Step 1: Intermittent asthma | Step 2: Persistent Asthma: Daily Medication | Step 3: Persistent Asthma: Daily Medication | Step 4: Persistent Asthma: Daily Medication | Step 5: Persistent Asthma: Daily Medication | Step 6: Persistent Asthma: Daily Medication | |
| Preferred Treatment | Inhaled short–acting beta2–agonist (SABA) as needed | Low–dose inhaled corticosteroid (ICS) | 2 Options: Low–dose inhaled corticosteroid (ICS) plus inhaled long–acting beta2–agonist (LABA); Medium–dose inhaled corticosteroid (ICS) | Medium–dose inhaled corticosteroid (ICS) plus inhaled long–acting beta2–agonist (LABA) | High–dose inhaled corticosteroid (ICS) plus inhaled long–acting beta2–agonist (LABA) AND consider omalizumab for patients who have allergies. Note: Clinicians who administer immunotherapy or omalizumab should be prepared to treat anaphylaxis that may occur. | High–dose inhaled corticosteroid (ICS) plus inhaled long–acting beta2–agonist (LABA) plus oral corticosteroids AND consider omalizumab for patients who have allergies. Note: Before oral corticosteroids are introduced, a trial of high–dose ICS plus LABA plus either LTRA, theophyline, or zileuton, may be considered, although this approach has not been studied in clinical trials. Clinicians who administer immunotherapy or omalizumab should be prepared to treat anaphylaxis that may occur. |
| Alternative Treatment (If alternative treatment is used and response is inadequate, discontinue and use preferred treatment before stepping up.) | Cromolyn, leukotriene receptor antagonist (LTRA), or theophylline. Note: Theophylline is a less desirable alternative because of the need to monitor serum concentrations levels. | Low–dose inhaled corticosteroid (ICS) plus either leukotriene receptor antagonist (LTRA), theophylline, or zileuton. Note: Theophylline is a less desirable alternative because of the need to monitor serum concentrations levels. Zileuton is less desirable because of limited studies as adjunctive therapy and the need to monitor liver function. | Medium–dose inhaled corticosteroid (ICS) plus either leukotriene receptor antagonist (LTRA), theophylline, or zileuton. Note: Theophylline is a less desirable alternative because of the need to monitor serum concentrations levels. Zileuton is less desirable because of limited studies as adjunctive therapy and the need to monitor liver function. |
Immunotherapy
Consider subcutaneous allergen immunotherapy in steps 2 through 4 for patients who have persistent, allergic asthma. This is based on evidence for house–dust mites, animal dander, and pollen; evidence is weak or lacking for molds and cockroaches. Evidence is strongest for immunotherapy with single allergens. The role of allergy in asthma is greater in children than in adults.
Guidance on Quick–Relief Medication at All Steps
- Inhaled short–acting beta2–agonist (SABA) as needed for symptoms. The intensity of treatment depends on severity of symptoms: up to 3 treatments every 20 minutes as needed. Short course of oral systemic corticosteroids may be needed.
Azathioprine (Imuran or Azasan):
Azathioprine (AZA) is an immunosuppressant (IS). It is a purine analogue that interferes with DNA synthesis and inhibits cell proliferation. Its metabolite 6-mercaptopurine (MP) is inactivated by thiopurine methyltransferase (TPMT) through methylation. Approximately 11% of the population has reduced TPMT activity and 0.3% of the population has true deficiency of TPMT. In these patients, active 6-MP accumulates, which can lead to bone marrow toxicity and myelosuppression. Hence a pre-treatment TPMT enzymatic activity level should be tested. In addition to routine labs (CBC, BMP, LFT), we recommend a baseline pre-treatment test for TB (with Quantiferon Gold), Hep B and Hep C. AZA is given orally, usually once or twice a day after meals. The usual oral dose range is 2-3 mg/kg/d. The following titration schedule may be followed 1 mg/kg/day PO initially in single daily dose or divided q12hr; this may be increased by 0.5 mg/kg/day after 2-8 weeks, then by 0.5 mg/kg/day every 2-4 weeks; not to exceed 2.5 mg/kg/d. It may take up to 12 weeks before the full benefit can be gauged. We recommend routine blood tests (CBC, BMP, LFT) for toxicity monitoring every 2 wks while escalating therapy, every month x 3 and then every 3 months if stable. AZA may cause GI side effects such as nausea, vomiting and diarrhea. Hepatotoxicity, leukopenia and pancreatitis need to be cautioned against. Other adverse effects include alopecia, skin rash and interstitial pneumonitis. Toxicity may be affected by concurrent use of: alkylating agents, allopurinol, aminosalicylates, angiotensin converting enzyme inhibitors (ACEI), anticoagulants (argatroban, bivalirudin, dalteparin, enoxaparin, fondaparinux, heparin, warfarin), cyclosporine, etanercept, everolimus, hydroxychloroquine, infliximab, mercaptopurine, mycophenolate, sirolimus, sulfasalazine, tacrolimus, trimethoprim-sulfamethoxazole, and due to kidney disease. Live viral or bacterial vaccines are recommended against while on AZA. Female patients are counseled to avoid pregnancy (since this drug falls into Pregnancy category D: use in life-threatening emergencies when no safer drug available. There is positive evidence of human fetal risk). Patients are made aware to call their physicians for unusual fever/chills, rash, itching/swelling, muscle pain, bleeding or bruising; excessive tiredness; pale skin; headache; confusion; dizziness; fast heartbeat; difficulty sleeping; dark urine; yellowing eyes/skin, swelling/extra fluid around the abdomen; vomit that contains blood or looks like coffee grounds; black stools; weakness; shortness of breath; and sore throat and other signs of infection. PJP prophylaxis is recommended if using AZA with another IS. AZA may increase the risk of progressive multifocal leukoencephalopathy (PML); a rare condition that cannot be treated, prevented, or cured and that usually causes death or severe disability. This typically presents with hemiparesis, apathy, confusion, cognitive deficiencies, and ataxia. Over long term, AZA may increase risk of developing certain types of cancer, especially skin cancer and lymphoma. Skin cancer risk can be mitigated with avoiding unnecessary or prolonged exposure to real and artificial sunlight (tanning beds, sunlamps, light therapy) and by wearing protective clothing, sunglasses, and sunscreen (with a SPF factor of 30 or above).
Modified Borg Rating Scale for Perceived Dyspnea
0 Nothing at all
0.5 Very, very slight shortness of breath
1 Very mild shortness of breath
2 Mild shortness of breath
3 Moderate shortness of breath or breathing difficulty
4 Somewhat severe
5 Strong or hard breathing
6
7 Severe shortness of breath or very hard breathing
8
9 Extremely severe
10 Shortness of breath so severe you need to stop
Drug: BENRALIZUMAB (FASENRA)
Treatment Plan: administer 30mg subcutaneously every 4 weeks for the first 3 doses followed by once every 8 weeks thereafter
Diagnosis: eosinophilic asthma
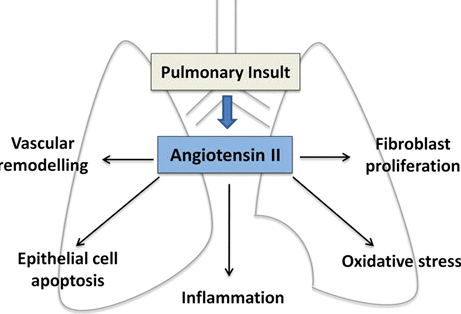
- Can losartan treatment modify the structural progression of COPD, particularly emphysema, and result in improved clinical outcomes?
Shrikrishna Et at Clinical Science 2012;123:487-498
Smoking cessation is the most important intervention for all COPD patients who smoke.
Physical Activity is recommended for all patients with COPD. We recommend a regular exercise regiment to achieve and maintain a maximal degree of musculoskeletal conditioning. Patients who achieve a maximal degree of musculoskeletal conditioning do better than those who do not for a given degree of pulmonary impairment.
Several studies have demonstrated benefit from Pulmonary Rehabilitation in patients with breathlessness (MMRC > 1, COPD GOLD Groups B,C,D) and following acute exacerbations. These benefits can be sustained after a single pulmonary rehabilitation program, especially if exercise training is maintained after the program is completed.
Oxygen therapy. We recommend oxygen therapy to maintain a saturation (Sp02) greater than 90% at all times, including rest, exertion, and sleep.
@NAME@ has a compelling environmental exposure for mold and fungus. Thus, we will refer @NAME@ to Occupational Medicine for further evaluation and recommendations regarding industrial and home hygiene evaluation and abatement.
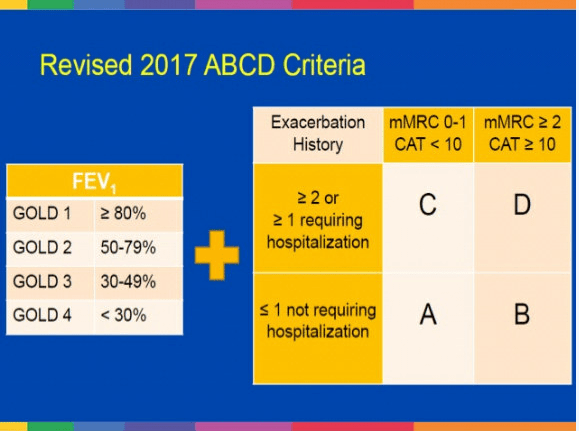
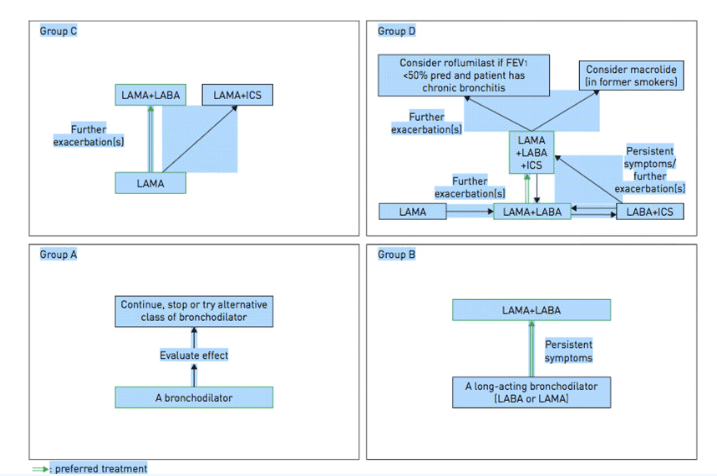
The appropriate medications for COPD depend on its stage of severity as determined by symptoms. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has proposed a strategy that is widely accepted. GOLD categorizes COPD severity as follows:
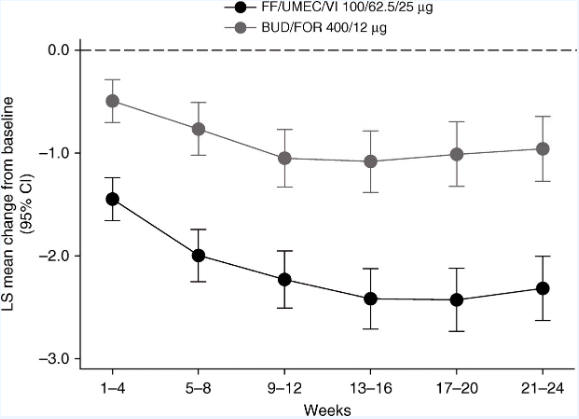
Triple therapy: Fulfill trial
FULFIL Trial: Once-Daily Triple Therapy for Patients with Chronic Obstructive Pulmonary Disease
David A. Lipson 1,2, Helen Barnacle 3, Ruby Birk 3, Noushin Brealey 3, Nicholas Locantore 1, David A. Lomas 4, Andrea Ludwig-Sengpiel 5, Rajat Mohindra 3*, Maggie Tabberer 3, Chang-Qing Zhu 3, and Steven J. Pascoe 1
+ Author Affiliations
Conclusions: These results support the benefits of single-inhaler triple therapy compared with ICS/LABA therapy in patients with advanced COPD.
The American College of Physicians has issued revised guidelines for COPD treatment, which include:
- In patients with stable COPD, reserve treatment for those who have respiratory symptoms and FEV1 of less than 60% of predicted.
- Patients with symptoms and FEV1 of less than 60% of predicted should be treated with long-acting inhaled beta-agonists, long-acting inhaled anticholinergics, or inhaled corticosteroids. Combination inhaled therapies may be used in these patients.
- Patients with COPD and resting hypoxia should be treated with oxygen therapy.
- Patients with symptoms and FEV1 of less than 50% of predicted should consider pulmonary rehabilitation.
- Alternative strategies for COPD
Today we discussed proceeding with application for Reslizumab (anti IL-5) we discussed risks benefits and alternatives.
What is CINQAIR (Reslizumab)?
CINQAIR is a prescription medicine used with other asthma med- icines for the maintenance treatment of asthma in people aged 18 years of age and older whose asthma is not controlled with the current asthma medicines
When added to other medicines for asthma, CINQAIR helps prevent severe asthma attacks (exacerbations) and can improve your breathing. Medicines such as CINQAIR reduce blood eosinophils. Eosinophils are a type of white blood cell that may contribute to your asthma.
Cinqair will be given in our infusion center with an IV 1 time every 4 weeks. It will take about 20 to 50 minutes to receive the full dose of CINQAIR.
The most common side effects include sore throat, muscle aches (chest pain, neck pain, extremity pain, muscle fatigue/pain) occasionally with elevated CPK levels (muscle enzyme) and allergic reactions.
In the research studies that lead to approval of Cinqair by the FDA, serious allergic reactions (anaphylaxis) happened in 0.3% (3 out of 1000 patients), typically within 20 minutes after infusion as early as the second dose
During the research studies cancers were found in 0.6% (6/1000) in those treated with Reslizumab and in 0.3 in placebo treated patients (3/1000)
Most cancers were found within the first 6 months of exposure
Patients were excluded from Cinqair/Reslizumab studies if they had evidence of a parasitic infection
It is not know if treatment with Cinqair would influence response to these infections. It is recommended to diagnose and treat andy parasite infections before starting therapy
We discussed data of steroid sparing potential of these agents in eosinophilic asthma and discussed that reported adverse effects as observed in the clinical trials include anaphylaxis and other hypersensitivity reactions (0.3%) , elevated muscle enzymes, oropharyngeal and musculoskeletal pain. Although the safety profile of these medications in the short term are reassuring, effects of long term treatment with anti IL5 has not been established.
Although these adverse events were not observed in clinical trials, we cannot completely exclude long term risks of malignancy, infection, or immunological adverse events. All questions answered.
Leave a comment